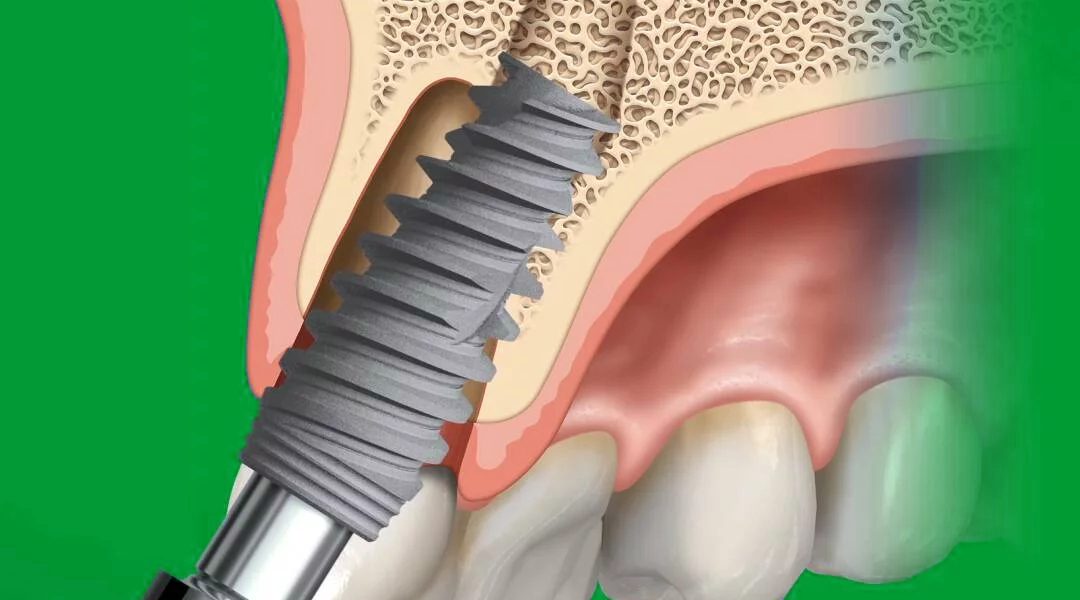
NobelActive™ is a dental implant system that originated from AlphaBioTEC Ltd., an Israeli dental implant company. AlphaBioTEC developed a self-drilling and bone-condensing implant design, and after presenting their research and clinical data, Nobel Biocare decided to collaborate and develop a new implant based on AlphaBioTEC’s design.
The development of NobelActive considered both surgical and restorative requirements. Clinicians wanted an implant system that offered maximum flexibility for placement and restorability, allowing options for two-stage, one-stage, and immediate loading procedures in all qualities of bone. Restorative clinicians wanted a variety of prosthetic options, including titanium and zirconia standard and custom abutments for different types of restorations on implants.
The design of NobelActive involved addressing challenges such as combining the back-tapered coronal portion with the need for titanium and zirconia fixed abutments and implant-level Procera® Implant Bridges. To achieve this, a conical prosthetic connection was chosen, providing a compact size, high strength, and a tight fit. The implant also featured a built-in Platform Shifting™ around the abutments, enabling cost savings for patients and eliminating the need for additional abutments for certain restorations.
Material selection was critical for ensuring the implant’s strength and performance. NobelActive used specially processed Grade 4 Titanium (MTA 009 and MTA 010) for its TiUnite® implants, which offered the required fatigue strength and thin cutting thread design.
Fatigue testing was conducted to evaluate the strength of the implant and abutment designs, and the endurance strength of the implant/abutment combinations was established to withstand occlusal loads. The torque strength was also considered during development, ensuring that NobelActive could tolerate the torque experienced during insertion.
The drilling protocol for NobelActive involved using its unique self-drilling thread design, allowing it to be inserted with straight twist drills without the need for bone taps. The drilling protocol was validated in a clinical study to ensure its efficacy in different bone qualities.
Before launching the NobelActive implant, Nobel Biocare conducted rigorous pre-clinical and clinical studies to validate the product’s safety and efficacy. Clinical studies demonstrated a high cumulative survival rate (CSR) for NobelActive implants, proving their long-term clinical success and safety for patients.
In conclusion, the development of NobelActive involved a comprehensive process of research, design, validation, and testing to create a versatile and successful dental implant system that meets the needs of both clinicians and patients.